Electrochemical Stock Illustrations – 267 Electrochemical Stock Illustrations, Vectors & Clipart - Dreamstime

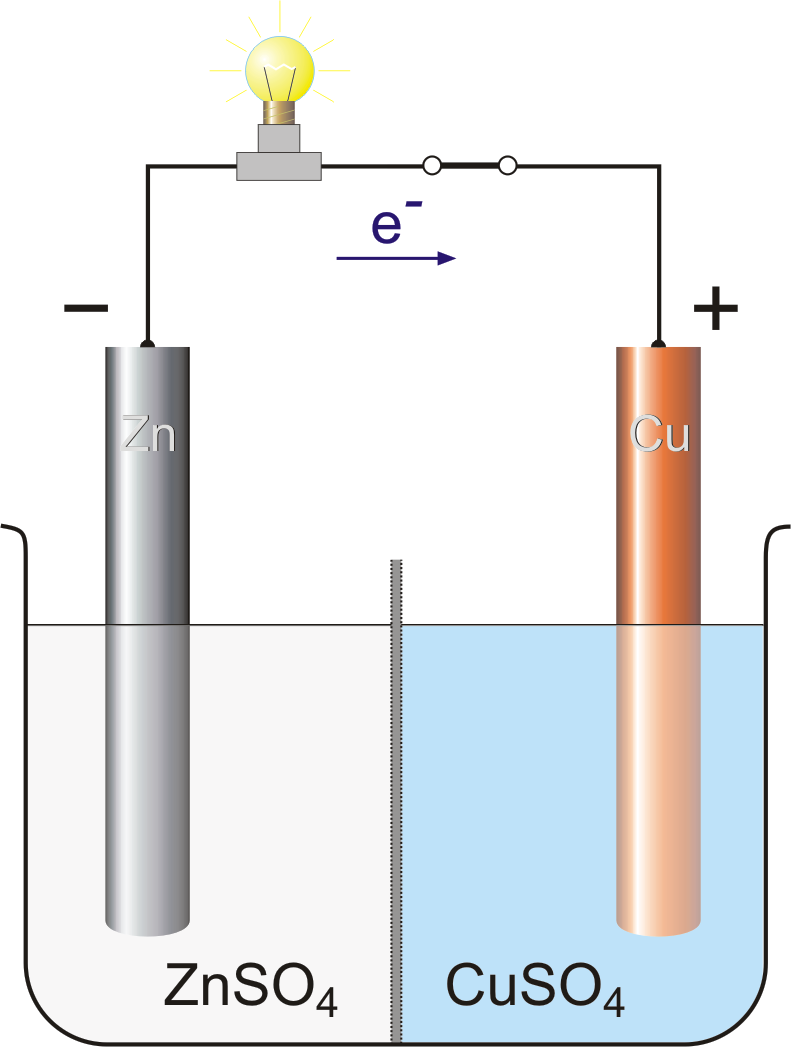
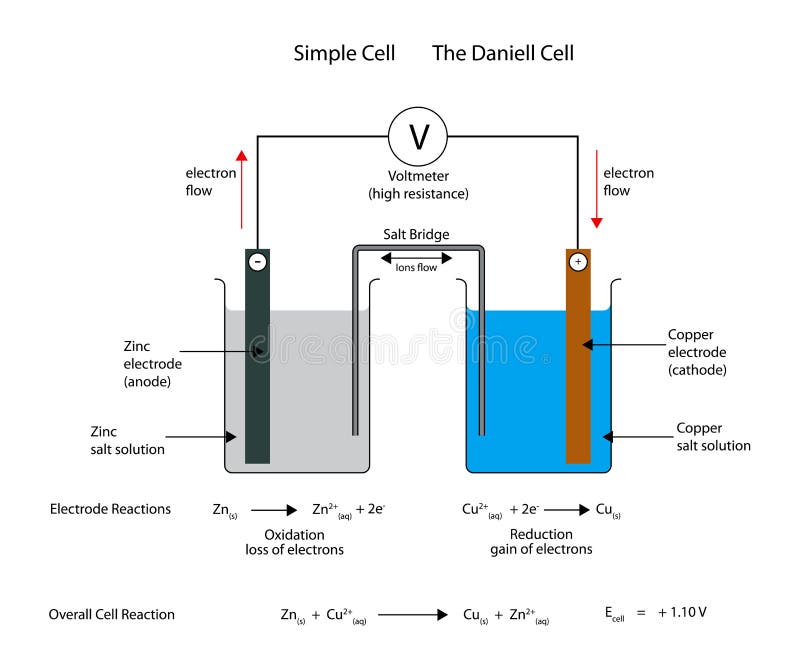
4 In the Daniell cell, instead of the salt bridge, a porous partition... | Download Scientific Diagram

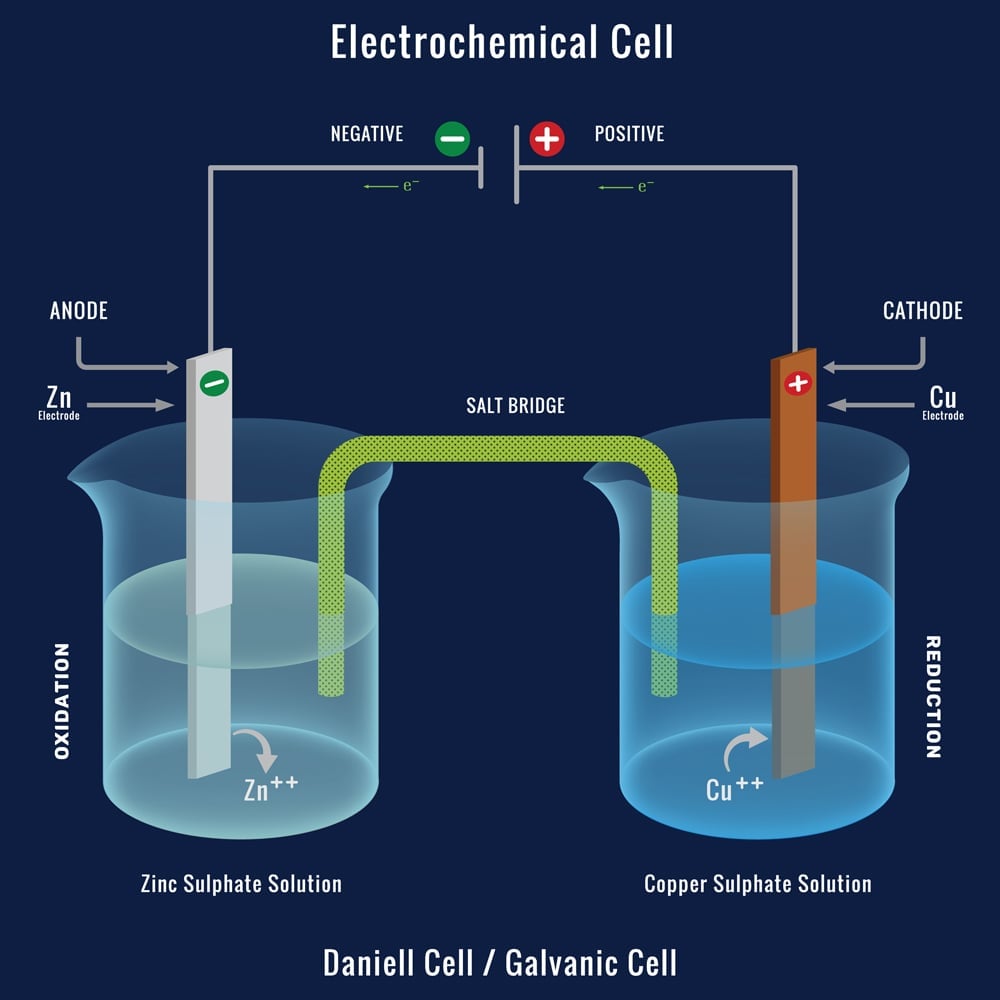
Electrochemistry -2 , galvanic cell,voltaic cell,daniell cell, salt bridge , making progress | Electrochemistry, Galvanic cell, Electrochemical cell

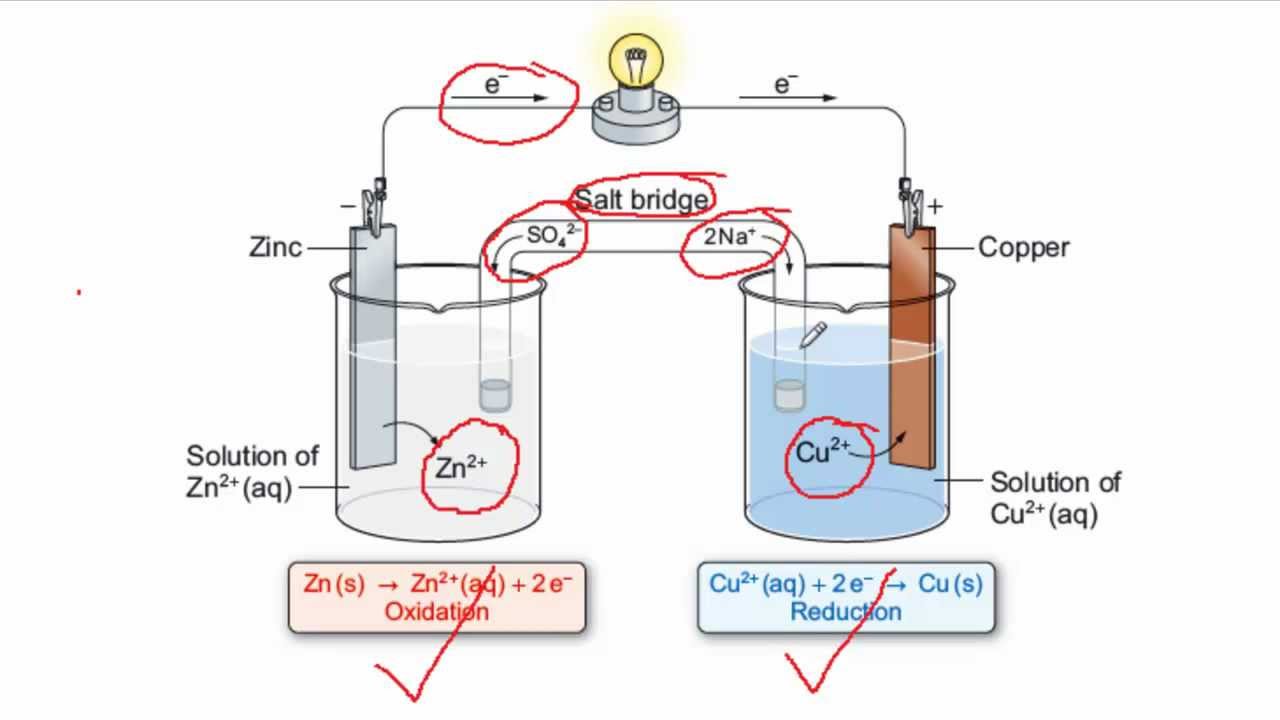
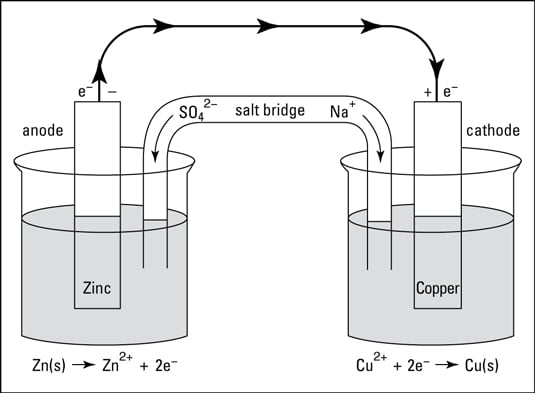
Draw a Daniell cell given the following cell notation: Pb|Pb(NO3)2||Ca|Ca(NO3)2 a. Label the electrolytes, electrodes, internal circuit, external circuit, and salt bridge (choose an appropriate salt). b. Write the oxidation half-reaction, the

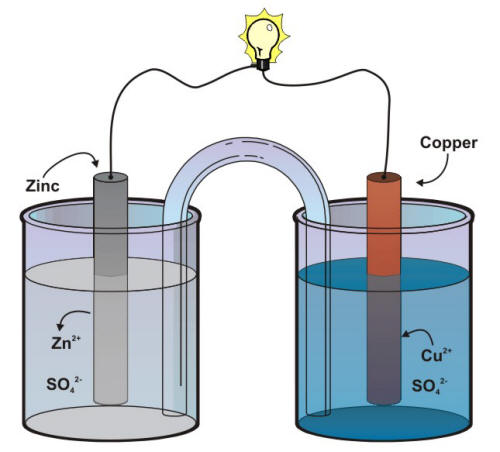
To Set up Simple Daniell Cell Using Salt Bridge and Determine its emf - Chemistry Practical Class 12 - Learn CBSE